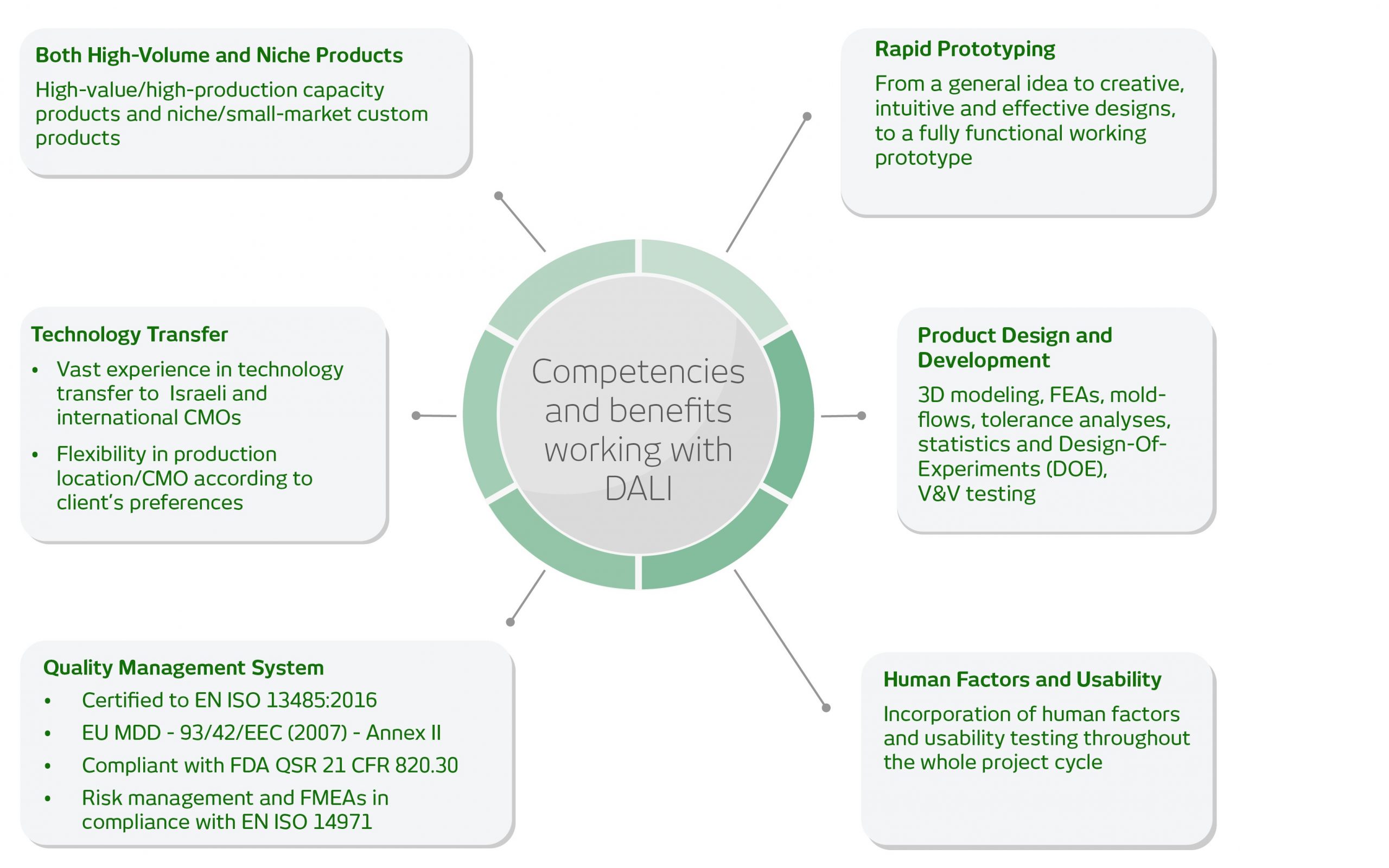
About DALI Medical Devices
DALI has been developing drug delivery devices from concept to commercialization for pharmaceutical and medical device companies since 2003. Our injectable drug delivery devices are designed for increased patient compliance to prescribed therapies (by Biologic/Biosimilar/Generic drugs), with greater ease and comfort than ever before. DALI’s creative and proven injectable drug delivery devices answer patient needs for easy self-administration.
Because we specialize in medical devices and drug delivery devices for the full product lifecycle we offer market-ready products as well as optional customization per specific needs. During the process, we include design and development, verification and validation testing, through all relevant standards and regulations.
ONE OF THE INJECTORS WE INVENTED AND DEVELOPED WAS LAUNCHED IN THE MARKET BY A MAJOR PHARMA COMPANY.
DALI’S NOVEL SAFETY NEEDLE – SAN®LIGHT – LAUNCHED WITH ADVANZ PHARMA’S MYTOLAC®/MYRELEZ® (LANREOTIDE) DRUG PRODUCT.
For more information Click here
For more information Click here
Management

David Daily
CEO and Co-Founder
Beginning in 1991, David acquired a broad range of experience in various R&D positions in the Israeli Defense Forces. From 1998 to 2003, while employed at Elan Medical Technologies Israel, a subsidiary of Elan Corporation, David led the development of a variety of drug delivery devices, including systems for disposable automatic injection and micro-infusion.
David received an MSc in Biomedical Engineering and a BSc in Mechanical Engineering from the Technion-Israel Institute of Technology. He also earned an Executive MBA with honors from the Hebrew University.

Lior Raday
CTO and Co-Founder
Before founding DALI, Lior worked as a senior product developer and testing engineer at Elan Medical Technologies Israel, which was a subsidiary of Elan Corporation. There, he was responsible for leading the design and development testing of a disposable automatic injection system. Before joining Elan, Lior worked as a control engineer at Ludan Engineering.
Lior holds a BSc in Mechanical Engineering from Ben-Gurion University.

Ziv Cahani
VP Business Development and Marketing
Ziv has over 15 years of experience in leading positions in Business Development, Product Management, Marketing, and R&D in a variety of industries. Prior to joining DALI, he worked in both startups and global corporations, such as Dip-Tech (acquired by Ferro), Siemens, and Radwin.
Ziv holds a BSc in Mechanical Engineering from Ben-Gurion University. He also received an MBA from the Interdisciplinary Center.

Dima Sarkorov
Director, R&D
Over six years at DALI Medical Devices, directing and overseeing design and development activities, from multiple innovative conceptual designs to final design for high volume production and verification testing.
Ensures project risk assessments are performed and appropriate actions are taken to protect the project plan and ensure objectives are met.
Liaise with Quality and Regulatory Assurance departments to ensure product development and validation is in accordance with the latest relevant global regulatory requirements and DALI’s QMS.
Dima holds an MSc in Mechanical Engineering from Tel Aviv University.

Yana Shmilovitz
Director, QA-RA
Yana Shmilovitz holds 14 years of experience in pharma and medical devices companies in QA positions.
Experienced in compliance with the medical device regulatory requirements: ISO, FDA, MDSAP, EUMDR.
Former Quality Engineer for manufacturing processes at Optonol, Alcon.
Former Quality Engineer for manufacturing processes at Omrix Pharmaceuticals, a J&J Company.
.

Elyasaf Laybovitch
Director, Operations
Elyasaf carries 7 years experience in drug delivery device combination products from concept to market. Guiding and managing CMOs inland and overseas to meet CE and FDA standards with full traceability of Product Life Cycle.
Establishing production lines with QC Plan, Master Validation Plan, and Device Master Record at CMOs.
Former R&D and NPI project manager at DALI.
Elyasaf holds an MSc in Biomedical Engineering from Tel Aviv University.
.

Haim Gil-Ad
Board Director
Haim carries 40 years of experience in the management of both governmental and private enterprise high-tech & biotech companies. Haim is pushing change management, innovative business & technology toward breakthroughs & success. In the last 17 years, after retiring from long service in the Ministry of defense, Haim led commercial high-tech and biotech companies from their infancy to business fruition. Haim is the founder of NovellusDx (currently FORE.Bio), leads the life science sector in the Merage Foundation, has board positions in a few biotech and high-tech companies, and empowers early-stage entrepreneurs around the globe, mainly in developing countries.